
SMART101 by SMART IMMUNE
The MEARY Center is in charge of the manufacturing of SMART101, developed by Smart Immune, as part of two Phase I/II clinical trials.
These trials are evaluating the safety and efficacy of SMART101 in accelerating immune reconstitution to fight infection and relapse after allogeneic hematopoietic stem cell transplantation.
Project phases
About us
Smart Immune is a clinical-stage biotechnology company developing ProTcell, a thymus-empowered T-cell progenitor therapy platform to fully and rapidly re-arm the immune system, enabling next-generation allogeneic T-cell therapies for all. The company aims to radically improve outcomes for patients with life-threatening diseases such as high-risk blood cancers and primary immunodeficiencies.
Smart Immune has ongoing collaborations with leading institutions in the US and Europe. ProTcell is already in three Phase I/II clinical trials looking at the acceleration of complete immune recovery in patients fighting cancer and infection and undergoing allogeneic hematopoietic stem cell transplantation.
Smart Immune is also developing therapies using gene-modified T-cell progenitors through its ProTcell platform to provide targeted treatments against cancer or AIDS, like persistent off-the-shelf CAR T-cells or HIV-resistant T-cells.
About SMART101 and the ProTcell platform
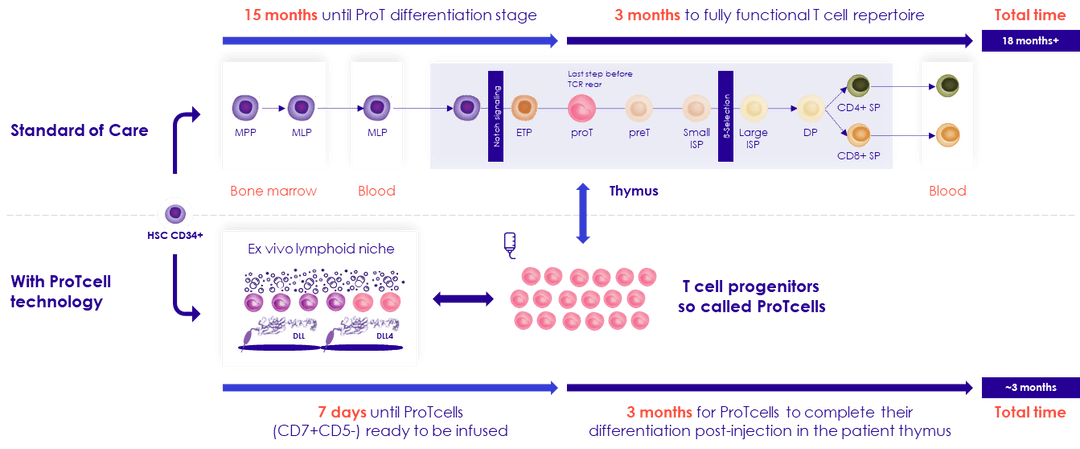
SMART101 is manufactured ex-vivo in 7 days from allogeneic peripheral blood stem cells, using Smart Immune's ProTcell T cell progenitor therapy platform. Once injected into the patient, SMART101 human T cell progenitors migrate to the thymus, where they are educated to become fully functional, self-tolerant T cells.
Preclinical data suggest that the ProTcell platform could reconstitute the immune system in 100 days instead of the 12 to 18 months usually observed, thus protecting patients from infection and relapse.
SMART101 is being produced by MEARY Center in two clinical trials, Smart Immune's development partner in France.
As part of this partnership, MEARY Center is participating in the clinical scale-up of the production of such a drug, the aim being for Smart Immune to be able to treat as many patients as possible by optimizing the manufacturing process for these cells.
ProTcell Platform
Clinical trial phase I/II (NCT04959903)
The Phase I/II trial (NCT04959903) is the first to be approved by the Food and Drug Administration (FDA). It is a multicenter, open-label study designed to enroll up to 36 adult and pediatric patients with hematological malignancies. It is designed to evaluate the safety and potential of SMART101 to improve clinical outcomes in the context of allogeneic hematopoietic stem cell transplantation.
The first patient included received an injection of SMART101 in December 2022.
Clinical trial phase I/II (NCT05768035)
A second European Phase I/II trial (NCT05768035) involving 34 adult patients will also assess the safety and efficacy of SMART101 in haploidentical hematopoietic stem cell transplants.
